Individualized for you.
Meet Cala kIQ™ — the only FDA-cleared, wearable device that delivers effective therapy for action hand tremor in people with essential tremor and Parkinson’s disease.
kIQ, pronounced “kick,” stands for Kinetic [movement] + IQ [smart]

Answer a few questions and find out if the Cala kIQ System could work for you.
Proven effective.
The Cala kIQ™ System with TAPS (Transcutaneous Afferent Patterned Stimulation) therapy is physician prescribed and clinically proven to reduce your action hand tremors.

Spiral drawing test before therapy session1
People experience tremor reduction with a complete 40-minute session3
Most maintain relief lasting for over an hour after therapy3*
1. Lin PT, et al. Noninvasive neuromodulation in essential tremor demonstrates relief in a sham-controlled pilot trial. Movement Disord 2018. Jul;33(7):1182-1183
2. Yu, J.Y., et al. Transcutaneous afferent patterned stimulation therapy reduces hand tremor for one hour in essential tremor patients. Frontiers in Neuroscience, 14. 2020.
3. Isaacson SH, et al. Prospective Home-use Study on Non-Invasive Neuromodulation Therapy for Essential Tremor. Tremor J. 2020;10:29. As measured by Cala device following three months of repeated home use in 205 patients with essential tremor who completed the study. Many participants were also taking medication for their tremor and it was difficult to assess the effect of the device compared to medication.
*SD 138min; median 60 min.
Be empowered.
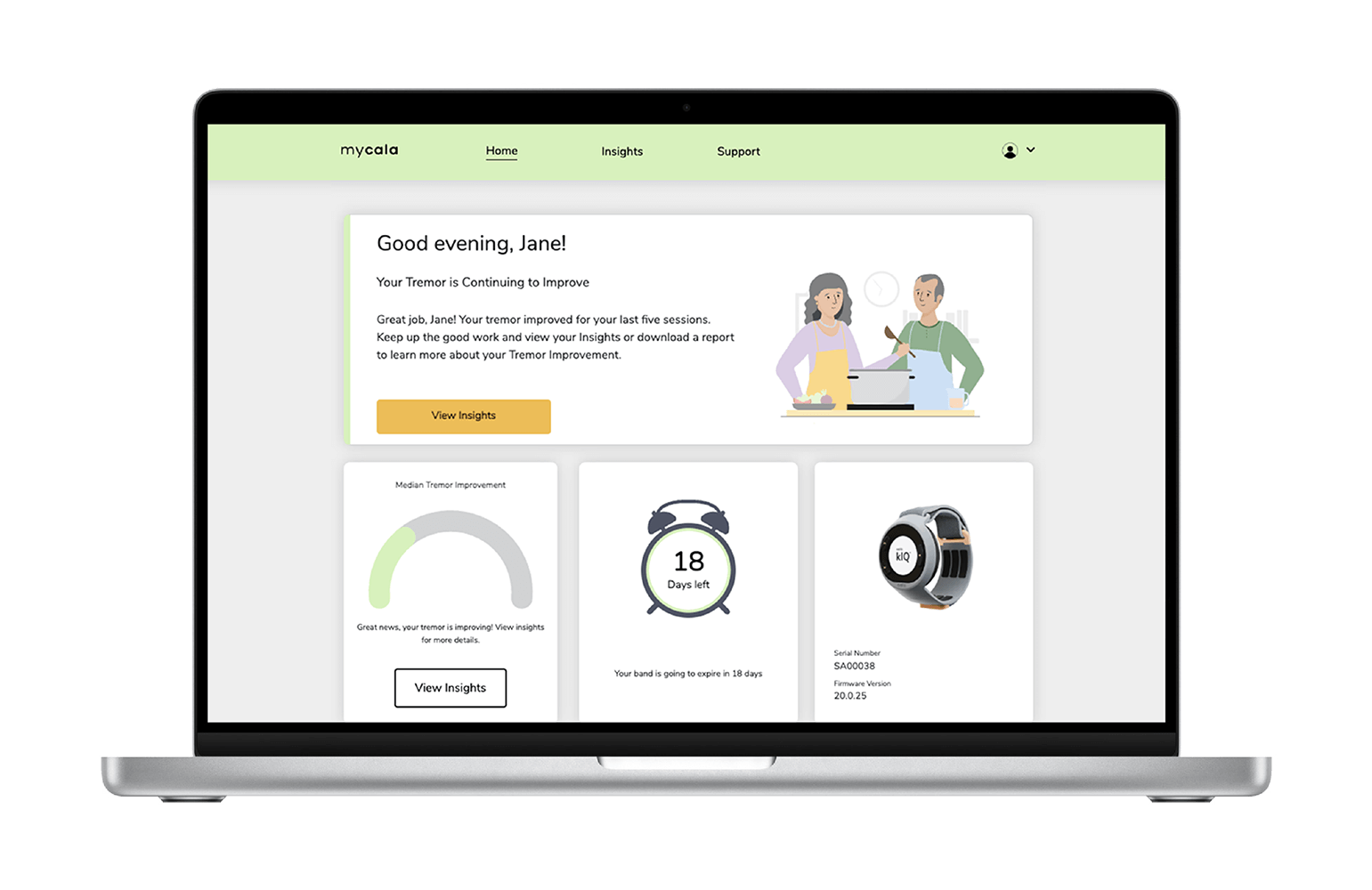
The Cala kIQ™ System empowers you with on-demand therapy and data insights to see your results over time.
See your secure therapy data on the MyCala.com patient portal.
Share a report of your results with your clinician.
Find resources for ongoing product support.
Safety information.
Review the Cala kIQ device’s safety information for more on indications for use, warnings, and side effects.
Real people. Real results.
Thousands of people are using Cala TAPS therapy. Hear from real users how it has made a difference.
How much does The Cala kIQ™ System cost?
The Cala kIQ System is reimbursed by the VA and many major commercial and Medicare Advantage plans. We work with you to understand your benefits and out-of-pocket costs, so you can make an informed decision about how Cala’s therapy fits into your treatment plan.
Try the Cala kIQ™ System
See if it’s right for you.
Talk to your healthcare provider to see if the Cala kIQ System is right for you. Your provider will assess your hand tremor and will help complete the necessary forms. Depending on your insurance, you may qualify for a free trial.
Got a prescription?
